Abstract
Mutations in SPTB gene coding β-spectrin can lead to congenital hemolytic anemia (HA) or red cell membranopathy. Most patients presented with mild to moderate HA with either spherocytes or ovalo-elliptocytes. Only one report in the literature described a family with non-immune hydrops fetalis (HF) due to homozygous SPTB: c.6055T>C (p.Ser2019Pro). Recently, we have identified the second patient presented with HF in Thailand with the same genotypes through genomic approach. This finding has driven extensive study to determine the impact of SPTB mutation in our cohort of unexplained HA and HF.
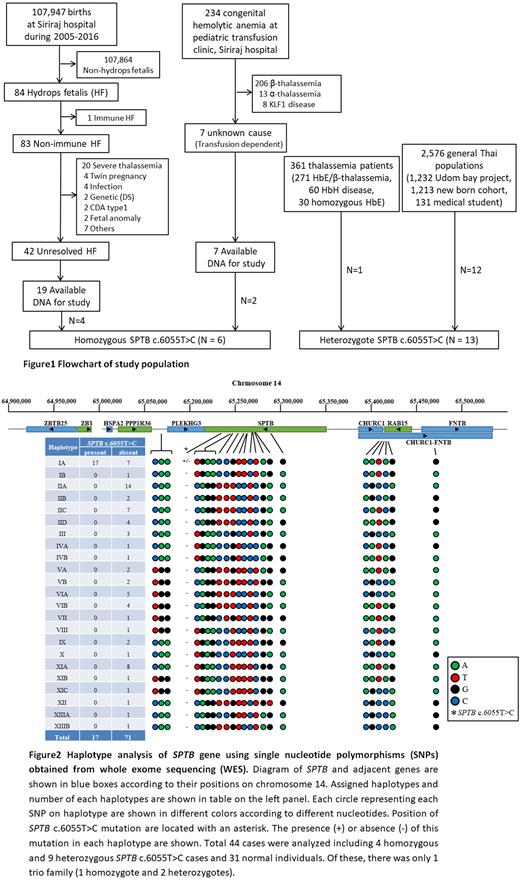
From 107,947 live births at our center between 2005 and 2016, 83 non-immune HF were identified. Nineteen cases from 42 unexplained HF (45%) had FFPE DNA available from autopsy (Fig 1). In addition, 7 patients who were transfusion dependent anemia due to unknown cause were recruited. These were subjects of our Whole Exome Analysis (WES) using Illumina HiSeq4000 capturing by SureSelect V5 plus UTR covering 21,522 genes. Moreover, we studied WES in 120 Thai individuals without anemia as controls. Surprisingly, 6 out of 26 patients (23%) were homozygous SPTB: c.6055T>C and none in controls. We confirmed this finding by direct genomic Sanger sequencing and set up a PCR-RFLP to detect this further in other cohorts. Interestingly, we found additional 13 heterozygotes of this mutation in our thalassemia patients (1 in 361) and general population (12 in 2,576) (Fig 1) making an unexpectedly high carrier rate of this "so-called rare genetic disease" into 0.47% in Thai population. Using Hardy-Weinberg principle, homozygous SPTB: c.6055T>C would be 5.42 per million or expected 358 cases in Thailand.
Since most reported SPTB mutations are sporadic or familial specific, we wondered whether this affected allele has a single or multiple origins. We generated a haplotype analysis of SPTB on chromosome 14q23 using 23 informative SNPs at the gene locus and adjacent region from WES data. Total 23 different haplotypes were generated and the SPTB c.6055T>C originated from the same ancestral chromosome as it was found only in haplotype IA (Fig 2). Therefore, we proposed to name this as spectrin Thai, instead of spectrin Providence which was named after the place of the first case reported (Gallagher PG, et al. J Clin Invest 1995), but not related to its native population.
All 4 survived patients due to homozygous spectrin Thai (one received intrauterine transfusion) had severe anemia at birth and neonatal jaundice requiring therapy and became transfusion dependent afterward. All had marked hepatosplenomegaly with blood picture of pyropoikilocytosis. Three patients underwent splenectomy without improvement on anemia, this finding contradicted those in patients with red cell membranopathies. Of note, hematology from 25 heterozygotes including 12 parents showed no apparently abnormal indices and normal EMA binding assay. Using shortgun proteomics to analyze red cell membrane proteins, hemolysate and plasma proteins, the only difference was we could detect unbound free β-spectrin in plasma from controls but not from spectrin Thai traits. This might be useful for future screening of heterozygotes at population level.
To explore whether red cells with spectrin Thai trait has any protection against malaria, the most important selective pressure in the past of our region we studied in vitro growth of P. falciparum and kinetics of erythroid invasion using live-cell microscopy. We found no significant differences between normal and spectrin Thai traits on these aspects. Since β-spectrin is involved on knob formation of malarial infected red cells that plays an important role on sequestration and cerebral manifestation, a further study using scanning electron microscopy comparing between malarial infected normal against spectrin Thai red cells is underway.
For the first time, we showed that red cell membranopathy might be endemic in the tropics than we expected. Homozygous spectrin Thai is one of the most common causes of unexplained HF and HA in our region. The possibility that red cell membranopathy traits might be under malarial pressure throughout human history has completed the whole red cell quantitative trait genetic polymorphism in our population. This is in consistent with those of hemoglobinopathy (thalassemia) and enzymopathy (G6PD), both are highly prevalent in our region due to the same biological advantage against malaria.
Viprakasit:Agios: Consultancy, Research Funding; Protagonist Therapeutics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.